What the FDA’s Phase-Out of Animal Models in mAbs Studies Means for Drug Discovery Labs
In a move that could reshape the landscape of preclinical drug development, the Food and Drug Administration (FDA) recently announced a plan to phase out its requirement for use of animal models in evaluating monoclonal antibodies (mAbs) and other drug candidates.
In a move that could reshape the landscape of preclinical drug development, the Food and Drug Administration (FDA) recently announced a plan to phase out its requirement for use of animal models in evaluating monoclonal antibodies (mAbs) and other drug candidates. For lab researchers and biotech innovators, this signals an exciting — and complex — shift in how safety and efficacy data can be generated and submitted.
But while this move promises to modernize and accelerate drug discovery through validated alternatives, it also raises important questions. Chief among them: Are we ready to fully replace animal models — and should we?
The National Association for Biomedical Research (NABR), a leading voice for the responsible use of animals in research, thinks this transition must be approached with both scientific rigor and caution. Let’s break it down.
What the FDA Announced — and Why It Matters
The FDA's announcement outlines a strategic roadmap to reduce animal use in preclinical studies through New Approach Methodologies (NAMs). These include:
- Organ-on-chip systems that simulate human organ function using microfluidics.
- In vitro assays using human-derived cell cultures and organoids.
- Computational models like AI-based toxicity prediction and physiologically based pharmacokinetic (PBPK) simulations.
The FDA’s position is clear: validated NAMs could reduce reliance on animals, cut costs, shorten development timelines, and better predict human responses — all without compromising safety.
What This Means for Lab Researchers
If you're working in drug discovery, this shift opens new opportunities:
- Regulatory Flexibility: The FDA encourages NAM data to be submitted alongside animal data in IND applications — particularly if your candidate is a mAb or human-specific biologic.
- Cost and Time Savings: NAMs like in vitro organoid models and in silico simulations may dramatically reduce the need for long, expensive animal studies.
- Better Predictive Tools: Organ chips and AI models might catch safety signals that animal models miss — improving your go/no-go decisions earlier in development.
But this also means investing in new infrastructure, training, and validation processes. The FDA's Roadmap encourages developers to adopt an integrative testing strategy — combining NAMs, historical human data, and any necessary reduced animal use in a weight-of-evidence framework.
NABR’s Take: Proceed with Caution, Not Abandonment
In response to the FDA’s announcement, NABR issued a press release welcoming scientific innovation but emphasizing that this is not a total departure from the use of animals — nor should it be. The FDA Modernization Act 2.0 expands the options available to researchers to prove safety and efficacy – it doesn’t remove the option to use an animal model. “NAMs must be validated and proven reliable before they can replace existing models,” says NABR President Matthew R. Bailey. “AI, for example, is only as good as the data it learns from — and much of that data still comes from animal studies.” (NABR Statement)
NABR stresses that animal models continue to provide indispensable insights into whole-body responses, metabolism, and immune function — areas where NAMs, at present, still fall short. For certain complex disease states and multi-organ interactions, living systems remain irreplaceable.
Striking a Scientific Balance
The core message from both the FDA and NABR is this: the future of preclinical testing is hybrid.
NAMs will grow in importance, especially for specific safety endpoints and human-specific interactions. But animal studies — especially those conducted with humane protocols and scientific oversight — will still play a vital role in answering questions NAMs cannot yet address.
For lab researchers, this means:
- Staying informed about FDA qualification pathways for NAMs.
- Participating in NAM validation studies — your data matters.
- Using animal models judiciously, where scientifically necessary and ethically defensible.
- Engaging early with regulators to design studies that meet evolving standards.
Final Thoughts
The FDA’s decision marks a pivotal moment in the evolution of drug development. It reflects growing confidence in 21st-century science and a desire to modernize our approach to drug safety — but it also challenges researchers to think critically about the tools they use and the assumptions they make.
As the regulatory framework shifts, so too must the research mindset: not toward a binary “animals or alternatives” debate, but toward a smarter, science-first approach that uses the best available tools — animal-based or otherwise — to deliver safe, effective medicines.
We’re Here to Help
From in vitro to in vivo, we’ve got you covered. HBio offers a number of in vitro solutions that can help your lab provide NAM data.
- Multiwell MEA systems can be used to accelerate screening.
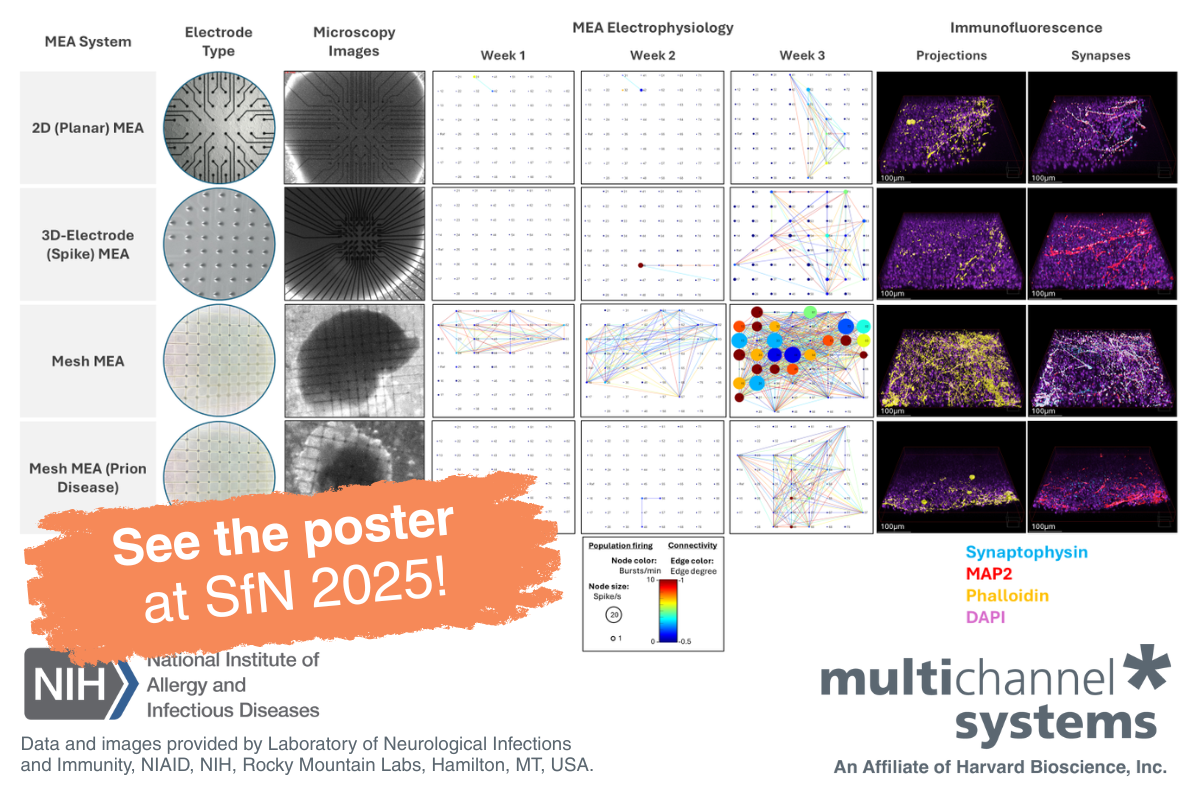
- Mesh MEA is a revolutionary new solution for organoid electrophysiology that allows researchers to record true-to-life data from inside an intact organoid without damage to its structure. As three-dimensional organoids tend to flatten and deform when placed on a traditional 2D MEA chip, the new Mesh MEA chip offers data that’s more physiologically relevant, as well as the possibility to conduct long-term experiments.
- The IntraCell laser-based optoporation system, used in conjunction with the MEA2100 Mini MEA system from Multi Channel Systems, utilizes an ultra-short pulsed laser to create transient nanopores and record intracellular action potentials without compromising cell viability. The system supports repeated recordings from the same cell for over 35 days and accommodates up to nine wells per experiment.
Contact us for more information on how we can support your drug discovery research.