Advancing Neuroscience: Leveraging Integrated Solutions for Breakthrough Discoveries
According to a recent study published by The Lancet Neurology with contributions from the World Health Organization (WHO), neurological conditions such as, dementia, epilepsy, stroke, and migraine affect more than three billion people worldwide, making these conditions the leading cause of illness and disability globally. Despite advances in neuroscience research, many of these conditions do not have fully effective treatments, making the development of innovative therapies a pressing need.
According to a recent study published by The Lancet Neurology with contributions from the World Health Organization (WHO), neurological conditions such as, dementia, epilepsy, stroke, and migraine affect more than three billion people worldwide, making these conditions the leading cause of illness and disability globally. Moreover, since 1990, the disability-adjusted life years (DALYs) caused by neurological conditions have increased by 18%, reflecting the growing impact of these diseases worldwide.1 Despite advances in neuroscience research, many of these conditions do not have fully effective treatments, making the development of innovative therapies a pressing need.
Harvard Bioscience (HB) is committed to supporting researchers in their quest to prevent, treat, and cure neurological diseases. By integrating various tools and solutions, researchers can achieve a holistic approach to neuroscience research. Harvard Bioscience solutions have been successfully combined in numerous studies to tackle complex neurological challenges, from studying neuronal activity to understanding disease mechanisms. The following examples showcase how researchers have used integrated methods to push the boundaries of neuroscience and generate impactful, reliable results.
The Power of Integrated Techniques: Examples from the Field
Integrated HB solutions allow researchers to easily validate results from in vitro models in a whole animal model, or vice versa, as well as to investigate interactions between different organ systems.
Alix is required for activity-dependent bulk endocytosis at brain synapses
Synaptic vesicle recycling during high-frequency stimulation involves a process known as activity-dependent bulk endocytosis (ADBE), crucial for maintaining synaptic function. This study aimed to investigate the role of the protein Alix in ADBE during physiological and pathological stimulations, such as epileptic seizures.
- Implantable telemetry from Data Sciences International (DSI) was used to monitor in vivo electroencephalogram (EEG) recordings in mice during kainate-induced status epilepticus (SE). This enabled real-time tracking of seizure frequency and propagation in Alix knockout (KO) and wild-type (WT) mice. The EEG data revealed that Alix knockout mice experienced significantly fewer seizures after SE induction compared to wild-type mice, despite no significant differences in the total duration of SE, thereby suggesting that Alix may be involved in facilitating seizure initiation rather than sustaining seizure duration.
- Whole-cell patch-clamp experiments were conducted using HEKA amplifiers to examine the functional impact of Alix deficiency on synaptic transmission in primary cultures of cortical and hippocampal neurons. These electrophysiological measurements demonstrated reduced frequency of spontaneous excitatory postsynaptic current (sEPSC), indicating impaired synaptic transmission.
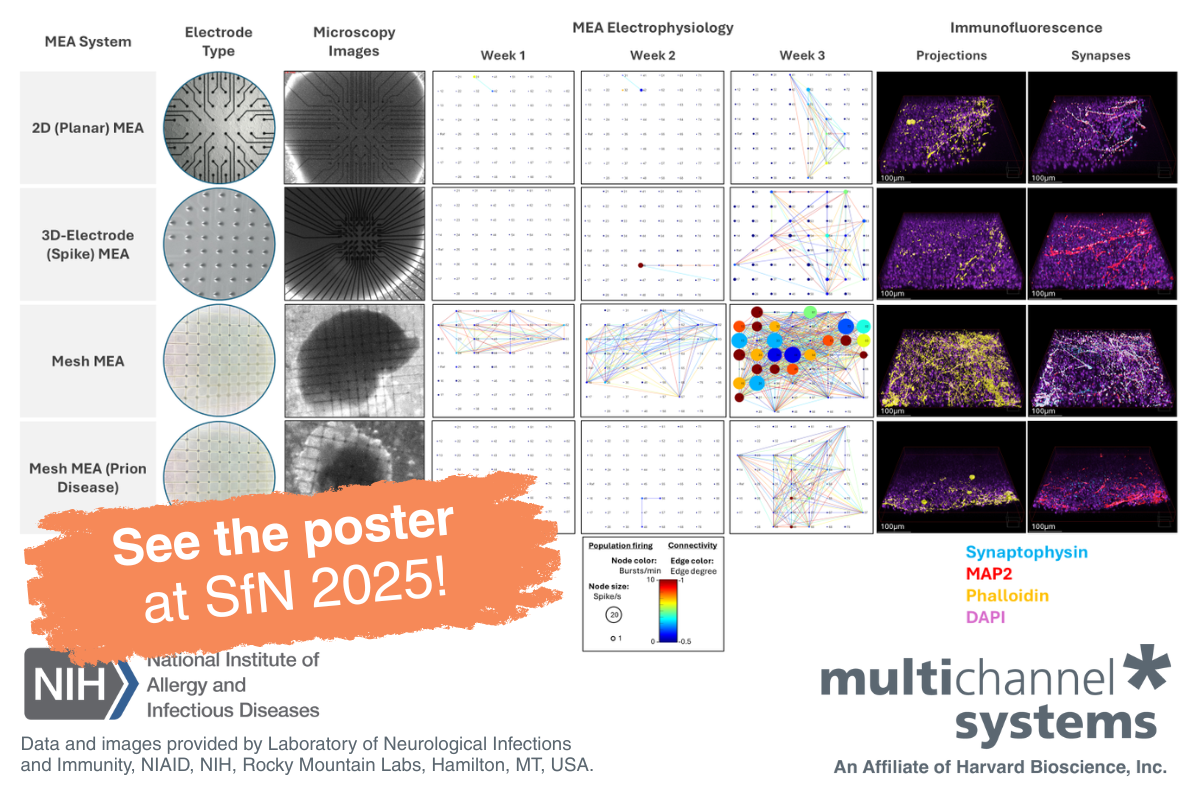
- Multielectrode array (MEA) recordings with a MEA-60 recording system from Multi Channel Systems were used to monitor network activity in cultured neurons and assess how Alix knockout affects spontaneous neuronal firing rates and burst behavior under high-frequency stimulation. In agreement with the patch clamp data, MEA data showed that the loss of Alix resulted in reduced frequency of bursts in hippocampal neurons during prolonged stimulation, confirming that Alix is critical for maintaining normal synaptic function during high-frequency neuronal activity.
This study shows Alix plays an essential role in ADBE, ensuring synaptic recovery during repetitive stimulation and supporting normal neuronal network activity.
Alzheimer's disease (AD) is associated with hippocampal hyperactivity and sleep disturbances in its early stages. This study explored the involvement of the melanin-concentrating hormone (MCH) system in hippocampal hyperactivity and its impact on sleep disturbances in an AD mouse model (AppNL-G-F).
- Whole-cell voltage clamp recordings were conducted on CA1 pyramidal neurons from hippocampal slices of AppNL-G-F mice, using HEKA amplifiers, to measure spontaneous excitatory postsynaptic currents. CA1 pyramidal neurons from AppNL-G-F mice exhibited elevated sEPSC frequencies, indicating an increased excitatory drive. Application of the MCH peptide downregulates excitatory synaptic transmission and restores neuronal activity to baseline levels, thereby demonstrating that MCH can reverse the aberrant synaptic activity seen in the AD model.
- Multielectrode array (MEA) plates (120MEA200/30iR-Ti) from Multi Channel Systems were used to monitor the mean firing rates (MFRs) of hippocampal neurons to test how MCH application modulates excitatory synaptic activity over time. These recordings demonstrated that the application of MCH peptide caused a sustained reduction in mean firing rates (MFRs) in cultured hippocampal neurons, indicating a decrease in neuronal excitability.
The study revealed that the MCH system plays a critical role in modulating hippocampal synaptic transmission and in firing rate homeostasis, highlighting its potential as a therapeutic target for mitigating some of the neural and sleep-related dysfunctions seen in AD.
Ischemic brain injury is associated with hypoxia and glucose deprivation, leading to neuronal death. Necroptosis, a programmed form of necrotic cell death mediated by RIPK1, is thought to be involved in ischemic injury, although its therapeutic potential requires further investigation.
- Multi-electrode array (MEA) recordings on the MEA60 (Multi Channel Systems) were used for non-invasive long-term registration of extracellular potentials of primary hippocampal cultures before and after hypoxia-induced injury, thereby providing insight into the functional integrity of neural networks. Hypoxic conditions led to a significant decrease in the number of network bursts and spikes, while treatment with the RIPK1 inhibitor Necrostatin-1 had a pronounced protective effect on the bioelectrical activity of hippocampal cultures. These results suggest that RIPK1 inhibition preserves the functional architecture of neural networks under ischemic conditions.
- The IR Actimeter from Panlab was employed to assess general locomotor activity, exploratory behavior, and anxiety to evaluate the animals' recovery following ischemic injury by tracking distance traveled, time spent in central and peripheral zones, and vertical movements. Mice subjected to ischemic injury showed reduced locomotor activity and increased anxiety-like behavior, as indicated by decreased distance traveled and more time spent in the peripheral zones. In contrast, mice treated with Necrostatin-1 showed improved motor activity and spent more time in the central zone, indicating a recovery in exploratory behavior and reduced anxiety, thus demonstrating increases the resistance to ischemic brain damage compared to untreated mice.
This study demonstrates that RIPK1 inhibition with Necrostatin-1 provides significant neuroprotection in both in vitro and in vivo models of ischemic injury, by preserving neural network activity and improving behavioral recovery, making it a promising therapeutic target for preventing neuronal necroptosis and mitigating ischemic brain damage.
Sleep-disordered breathing (SDB), such as obstructive sleep apnea, is a major risk factor for Alzheimer’s disease (AD), yet the mechanisms by which SDB contributes to AD pathology remain unclear. This study aimed to investigate the effects of SDB on AD pathology, using a novel mouse model that mimics key aspects of human SDB, including altered breathing during sleep, sleep disruption, moderate hypoxemia, and cognitive impairment.
- Electroencephalogram (EEG) and electromyography (EMG) signals were recorded continuously using DSI telemetry to monitor the sleep-wake cycles and, in combination with whole body plethysmography, assess breathing patterns in freely moving mice. Telemetric EEG recordings revealed disrupted sleep patterns in mice with SDB, with more frequent transitions between REM and non-REM (NREM) sleep, reduced NREM sleep, and shorter sleep bouts that resulted in a sleep debt, the equivalent of human daytime sleepiness.
- Whole body plethysmography (Buxco FinePointe Series WBP) from DSI was used to monitor key respiratory parameters, such as peak inspiratory flow (PIF), inspiratory time (EIP), and the frequency of breathing cycles, in unrestrained mice during both sleep and wake phases. Mice with cholinergic basal forebrain (cBF) lesions, which modeled SDB, exhibited disordered breathing patterns during sleep, including reduced breath frequency and increased variability in inspiratory flow and exhalation time. These breathing disruptions correlated with reduced blood oxygen saturation (SpO2), mimicking human obstructive sleep apnea.
This research paper demonstrated that sleep-disordered breathing exacerbates cognitive decline and AD pathology in a SDB mouse model, thereby suggesting that managing sleep-disordered breathing represents a candidate strategy for slowing the progression of Alzheimer’s disease.
Moreover, HB solutions provide researchers with tools to verify the clinical translatability of their preclinical model.
Central nervous system (CNS) drug discovery often faces high attrition rates in clinical trials due to the lack of translatable biomarkers between animal models and human studies. This study aimed to evaluate the mismatch negativity (MMN) response, an emerging EEG-based translational biomarker, in patients with schizophrenia (SZ), and back-translating these findings into animal models.
- Rats were subchronically exposed to phencyclidine (PCP) to induce a schizophrenia-like phenotype. DSI telemetry was then used to record electroencephalogram (EEG) signals of these freely moving animals during an auditory stimuli protocol, aiming to monitor MMN-like responses. In the PCP-treated rats, EEG recordings revealed a significant attenuation of the MMN response when compared to vehicle-treated controls, similar to the attenuation of MMN responses observed in human schizophrenia patients.
These findings demonstrate that DSI telemetry is a valuable tool for recording EEG-based biomarkers like MMN in animal models, allowing the back-translation of clinical findings into preclinical research. The similarity between the MMN impairments in PCP-treated rats and human schizophrenia patients supports the use of MMN as a translatable biomarker during both preclinical and clinical stages of SZ drug development.
A Holistic approach to Neuroscience Research
In the rapidly evolving field of neuroscience, breakthroughs are achieved by leveraging a diverse array of tools and techniques. Moreover, converging experimental evidence from multiple techniques strengthens research conclusions, thereby validating findings and providing a more robust understanding of brain function and neurological disorders.
Harvard Bioscience offers a comprehensive suite of solutions designed to meet your research needs, from high-throughput cellular applications to recording data in freely moving animals. These technologies enable researchers to integrate various methodologies, providing a more holistic approach to understanding complex neurological diseases and conditions.
Let’s chat!
Are you looking for solutions to enhance your neuroscience research? Are you interested in learning more about one or more techniques highlighted in this article?
Contact us today, and our team will connect with you!
References
1World Health Organization. Over 1 in 3 people affected by neurological conditions, the leading cause of illness and disability worldwide. 14 March 2024. https://www.who.int/news/item/14-03-2024-over-1-in-3-people-affected-by-neurological-conditions--the-leading-cause-of-illness-and-disability-worldwide