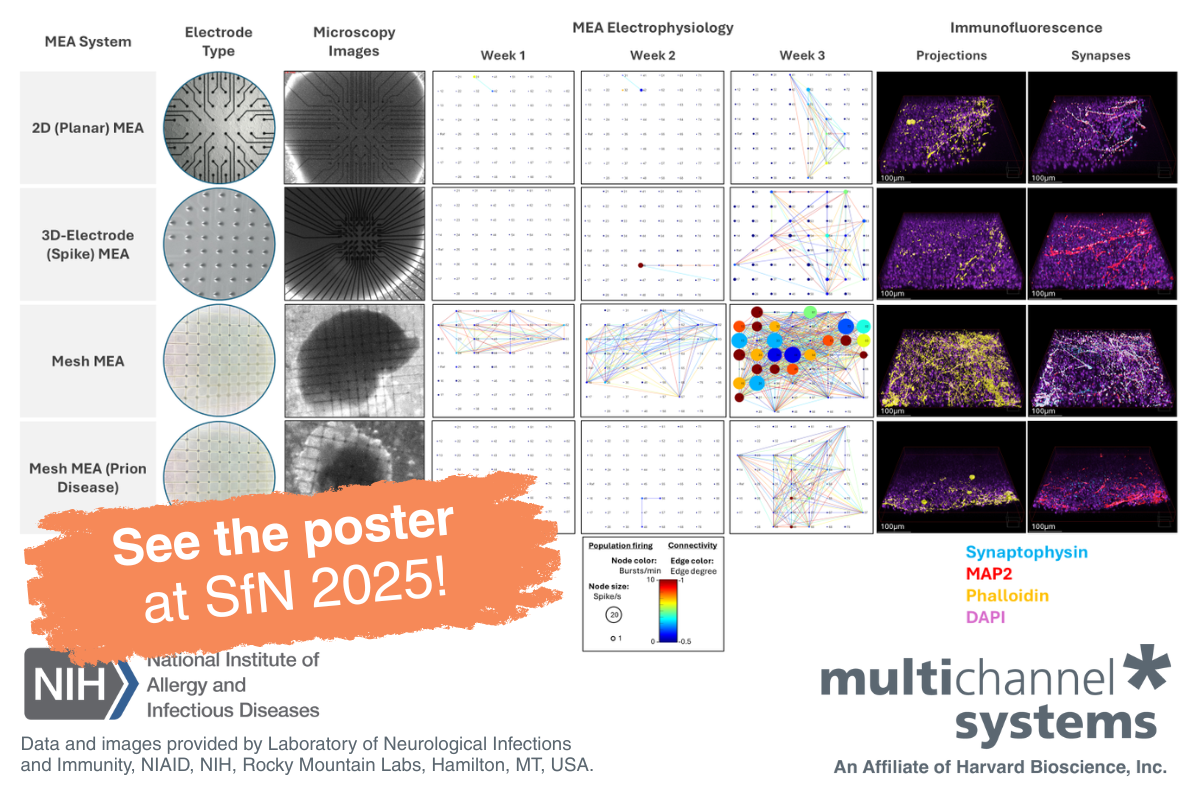
Modeling Prion Disease and Neural Connectivity Using Mesh MEA
For this study, mature cerebral organoids (6-7 months old) were transferred to a Mesh MEA using a polyethyleneimine and laminin coating protocol and BrainPhys cell culture media. After 3 weeks in culture, COs displayed higher electrical activity on the Mesh MEA compared to planar MEA chips, which contained either flat or 3D-spike electrodes.
Organoids are three-dimensional tissues generated from stem cells, and they’ve become an essential research tool for modeling human (patho)physiology in vitro, particularly in the context of neurological disorders. For example, brain organoids recapitulate aspects of human brain development and function, which allows them to offer a framework by which the cellular and network-level mechanisms underlying conditions such as epilepsy, Alzheimer’s disease, or prion diseases can be studied.
Multielectrode arrays (MEAs, also called “microelectrode arrays”) are the most widely adopted technology for electrophysiological assessments in organoids, but MEA platforms were largely developed before organoids emerged as experimental models. As a result, current MEA system designs are not ideal for electrophysiological recordings in organoids. Using brain organoids on planar MEA chips (which contain either flat or 3D-spike electrodes) leads to structural deformation of the organoids and a loss of electrophysiological complexity due to changes in morphology. Consequently, high-fidelity and long-term recordings are compromised.
To overcome these challenges and offer a more reliable platform for electrophysiology studies in organoids, Multi Channel Systems (an affiliate of Harvard Bioscience), in collaboration with partners at NMI, developed Mesh MEA. This novel, grid-scaffold MEA chip is designed to preserve 3D tissue structures long-term, thus refining electrophysiological data collection.
Preserving Organoid Architecture and Function with Mesh MEA
Cerebral organoids (CO) cultured on the Mesh MEA demonstrated sustained electrical activity, more intact architecture of neuronal projections, and conserved synapse formation compared to organoids cultured on planar MEA, with either flat or 3D-spike electrodes (Figure 1).
For this study, mature CO (6-7 months old) were transferred to a Mesh MEA using a polyethyleneimine and laminin coating protocol and BrainPhys cell culture media. After 3 weeks in culture, COs displayed higher electrical activity on the Mesh MEA compared to planar MEA chips, which contained either flat or 3D-spike electrodes. Connectivity maps data(1) suggests that the scaffold Mesh MEA plays a role in preserving the electrophysiology of CO.
Moreover, immunofluorescence images, obtained after the last MEA recordings, showed that CO on the Mesh MEA expressed more synapses (synaptophysin-labeled) and a more intact architecture of neuronal projections (phalloidin-labeled) compared to both planar MEA chips (flat or 3D-spike electrodes). Immunofluorescence indicates that Mesh MEA preserved the 3D architecture of neuronal projections and maintains healthy levels of synapses, which are compromised, to some degree, in both planar MEA chips.
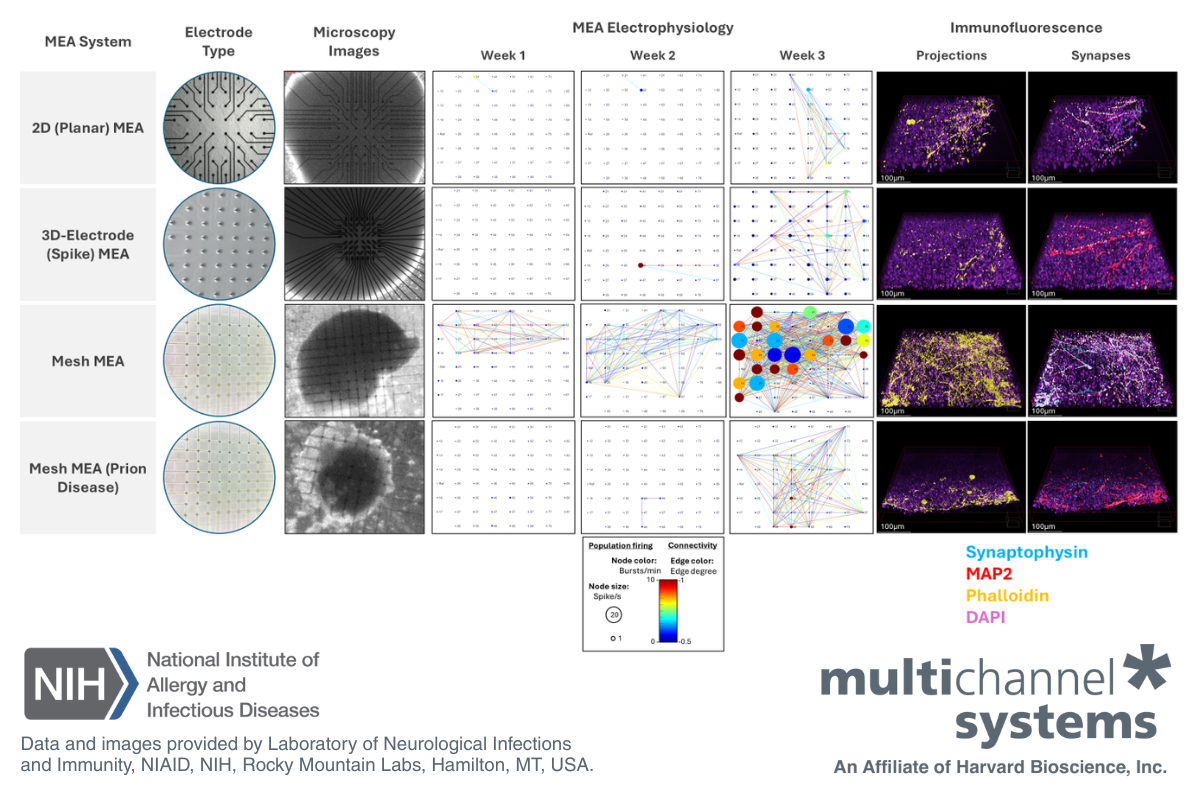
Figure 1: Mesh MEA conserves organoid structure and electrical activity. Data from wild-type (WT) organoids culture on planar MEA, including flat electrodes (first row), 3D-spike electrodes (second row), and Mesh MEA (third row). The fourth row displays data from an organoid harboring a mutation within the prion protein. Microscopy images of the electrodes in each chip type and the cultured organoids are shown in the second and third columns, respectively. Connectivity maps (columns 4 to 6) represent the electrical activity of the organoids at weeks 1, 2, and 3. The connectivity map displays spike rate as node size, burst rate as node color, and network events (as lines) between electrodes. 3D immunofluorescence images, taken at the outer layer of the organoid, exhibit higher expression of phalloidin (in gold; column 7) and synaptophysin (in turquoise; column 8). DAPI (in pink) and MAP2 (in red) were used as reference markers (merge). Legends for data and image interpretation are shown at the bottom of the figure. Data courtesy of the Prion Cell Biology Section, Laboratory of Neurological Infections and Immunity, NIAID, NIH, Rocky Mountain Labs, Hamilton, MT, USA.
Application of Mesh MEA in Prion Disease Modeling
NIH/NIAID researchers utilized Mesh MEA to study CO with a prion protein mutation(2) that causes human prion disease. Long-term monitoring of network activity in live CO on the Mesh MEA suggests that the mutation causes neuronal network breakdown associated with oxidative stress. Moreover, proteomic and metabolomic analyses link the decrease in neuronal network activity to vesicle transport and neurotransmitter trafficking and usage, which are distinct from the loss of function of the cellular prion protein.
To learn more about this research, visit Simote Foliaki, PhD, at poster number LBP152 during the Society for Neuroscience (SfN) 2025 meeting in San Diego, CA. You can also meet him at the Harvard Bioscience booth (Booth #1928) on Tuesday, November 18 from 1pm - 3pm for a Meet the Researcher session, where he'll be available for questions and chats about his work. Learn more about visiting Harvard Bioscience at Neuroscience 2025.

Conclusion
Mesh MEA technology empowers researchers to examine the complexity of brain organoids in ways not attainable with planar MEA. Mesh MEA ability to preserve 3D tissue structures and enable long-term recordings provides a powerful platform for disease modeling, developmental biology, drug discovery, and translational research.
References
- Dastgheyb R. M. et al., Neuroinformatics. 2020 Jan;18(1):163-179. PMID: 31273627
- Foliaki S. T. et al., Mol Brain. 2021 Oct 11;14(1):156. PMID: 34635127
Acknowledgements
Harvard Bioscience, Inc. thanks Simote Foliaki, PhD, Bradley Groveman, PhD, and Cathryn Haigh, PhD (Prion Cell Biology Section, Laboratory of Neurological Infections and Immunity, NIAID, NIH, Rocky Mountain Labs, Hamilton, MT, USA) for sharing this data. And thanks as well to Peter Jones and his team from the Natural and Medical Sciences Institute (NMI), Reutlingen, Germany, for their work on the development of Mesh MEA.